DB Diagnostics LLC
WELLlife COVID-19/Influenza A&B Test
WELLlife COVID-19/Influenza A&B Test
For Emergency Use Authorization (EUA) only* | For in vitro diagnostic use
WELLlife™ COVID-19 / Influenza A&B Test utilizes advanced immunoassay technology to quickly and accurately differentiate between SARS-CoV-2, influenza A, and influenza B with a single nasal swab. Recommended for rapid diagnostics, this test is an essential tool for timely management of viral infections, supporting swift treatment decisions during the flu season and amidst public health concerns.
INTENDED USE:
The WELLlife™ COVID-19 / Influenza A&B Test is a lateral flow immunochromatographic assay intended for in vitro rapid, simultaneous qualitative detection and differentiation of influenza A and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly from anterior nasal swab specimens of individuals with signs and symptoms of respiratory infection consistent with COVID-19 within the first five (5) days of symptom onset when tested at least twice over three days with at least 48 hours between tests. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. § 263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
ACCURACY:
KEY BENEFITS:
- Cost-effective: Differentiate between SARS-COV-2, influenza A, and influenza B antigens with a single test using an anterior nasal swab specimen.
- Rapid Results: available in 10 minutes allows for testing and treatment decision-making during the same office visit.
- Quality Assurance: The outstanding clinical performance, including built-in kit controls for external quality testing, enables physicians to make quicker and more confident decisions.
- Extended Detection Window: Offers a broader detection window for differentiation of SARS-COV-2, influenza A, and influenza B antigens within five days of symptom onset.
- Wide Storage Temperature: 36°F-86°F (2°C-30°C) allows for easier product storage.
NUMBER OF TESTS: 25
CPT CODE(S): 87811QW (COVID-19), 87804QW (Flu A), 87804QW-59 (Flu B)
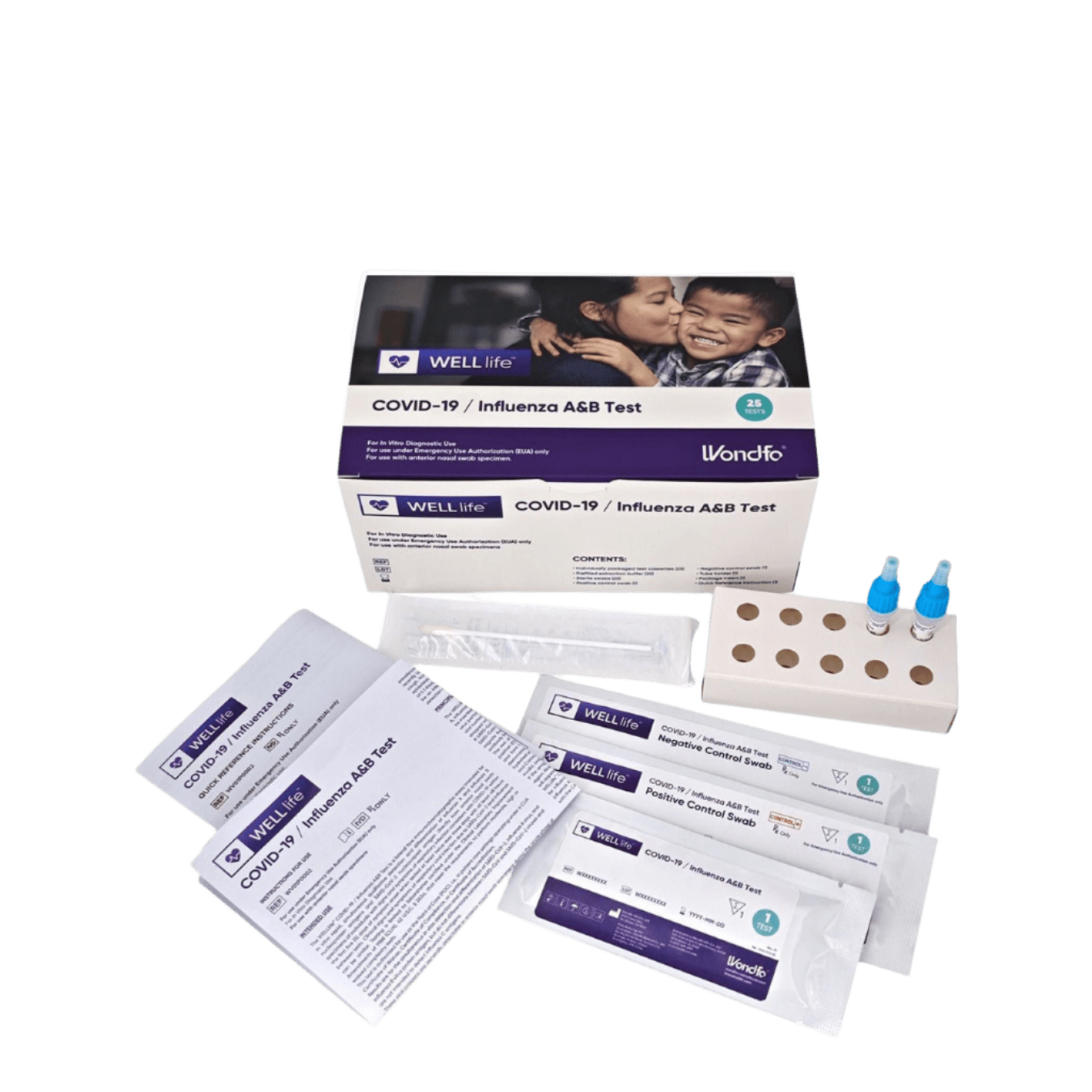
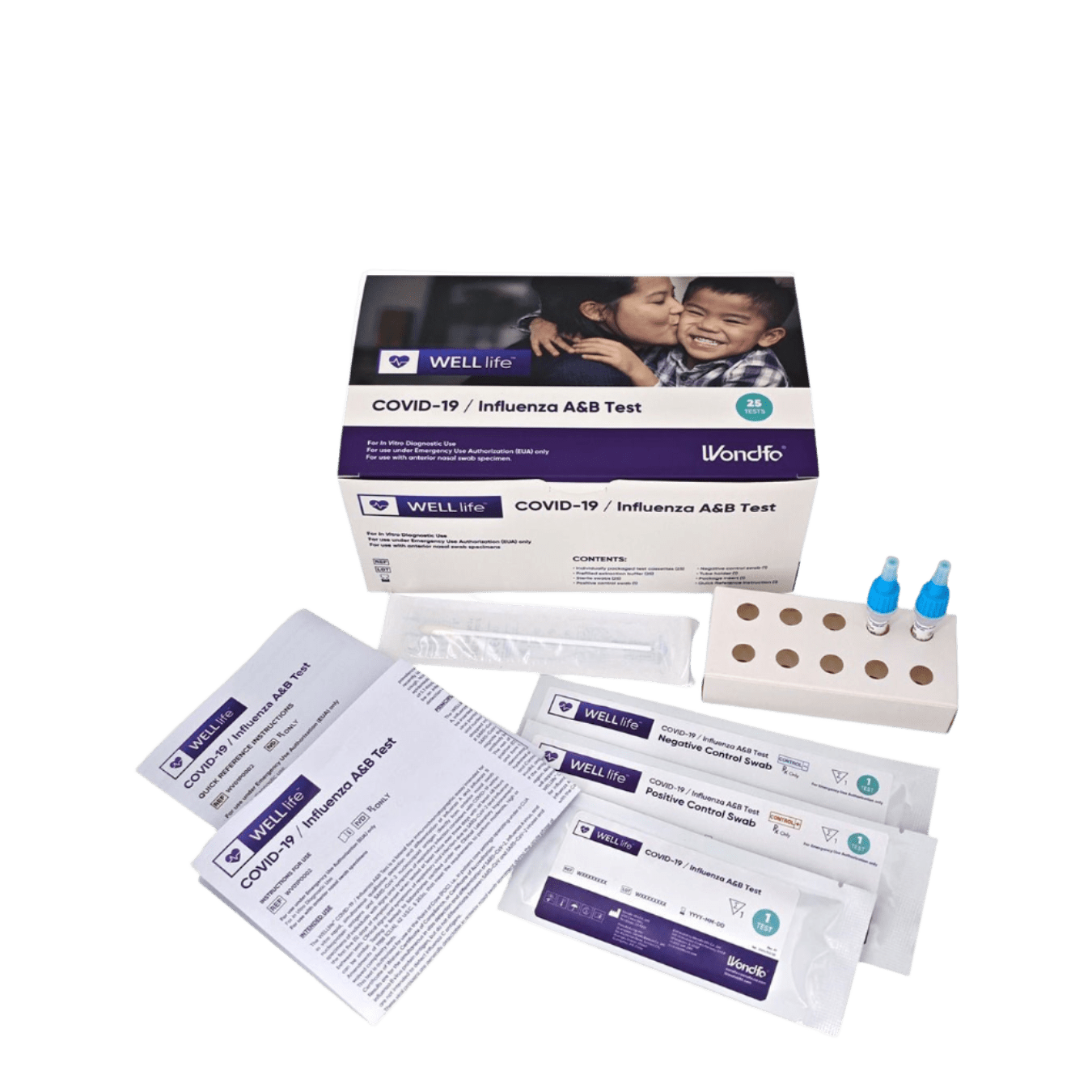
COMPONENTS IN 1 KIT:
- 25 WELLlife™ COVID-19 / Influenza A&B test cassettes containing CHO monoclonal antibodies to nucleocapsid protein of influenza A, influenza B and SARS-CoV-2 and containing 0.05% Proclin 300 as preservative
- 25 Prefilled extraction buffer tubes containing 400μL of Tris-HCl buffer with detergents and preservative (containing 0.05% Proclin 300 as preservative)
- 25 Sterile swabs
- 1 Positive control swab coated with non-infective influenza A, B and SARS-CoV-2 antigen (non-infectious recombinant nucleocapsid protein) and containing 0.05% Proclin 300 as preservative
- 1 Negative control swab coated with non-infectious inactivated Streptococcus Group A and containing 0.05% Proclin 300 as preservative
- 1 Tube holder (1)
- 1 Package Insert
- 1 Quick Reference Instructions (QRI)
DOCUMENTATION:
WELLlife COVID-19 Influenza A&B POC Package Insert
WELLlife COVID-19 Influenza A&B POC Quick Reference Instruction
WELLlife COVID-19 Influenza A&B POC Patient Fact Sheet
WELLlife COVID-19 Influenza A&B POC HCP Fact Sheet
Share