1
/
of
1
Verséa Diagnostics
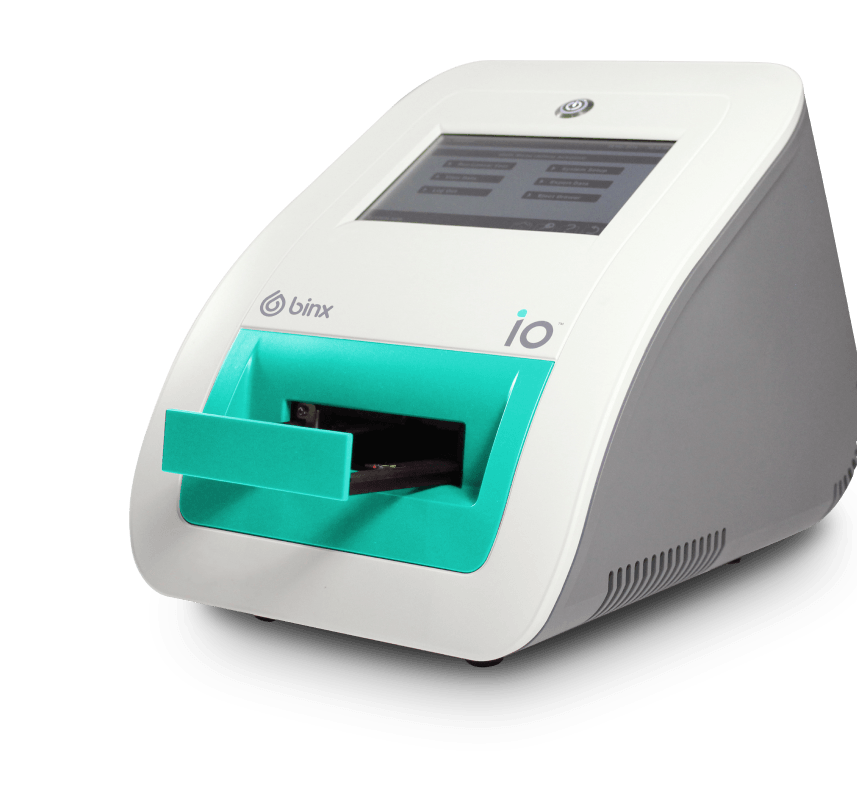
Binx io Instrument
Binx io Instrument
The FDA-cleared, CLIA-waived, rapid molecular POC platform offering Chlamydia and Gonorrhea PCR testing. Easy-to-use platform requires no calibration, preventative maintenance, or interpretation of results and can be used by non-lab-trained personnel.
* Easy to install and use.
* Fully-automated.
* Requires no calibration, preventative maintenance
* FDA-cleared and CLIA-waived
* For Point-of-Care molecular PCR testing
* Results available in under 30 minutes
* Enables single-visit “test and treat”
* Integrates with EMR/LIS
Contents:
1 io Instrument
1 Main Power Cable with Plug
1 External Power Supply
1 io Instrument Operator Manual
1 io Instrument Repackaging Instruction Sheet
* Easy to install and use.
* Fully-automated.
* Requires no calibration, preventative maintenance
* FDA-cleared and CLIA-waived
* For Point-of-Care molecular PCR testing
* Results available in under 30 minutes
* Enables single-visit “test and treat”
* Integrates with EMR/LIS
Contents:
1 io Instrument
1 Main Power Cable with Plug
1 External Power Supply
1 io Instrument Operator Manual
1 io Instrument Repackaging Instruction Sheet
Share