1
/
of
2
OraSure Technologies
OraQuick HCV 25 Count
OraQuick HCV 25 Count
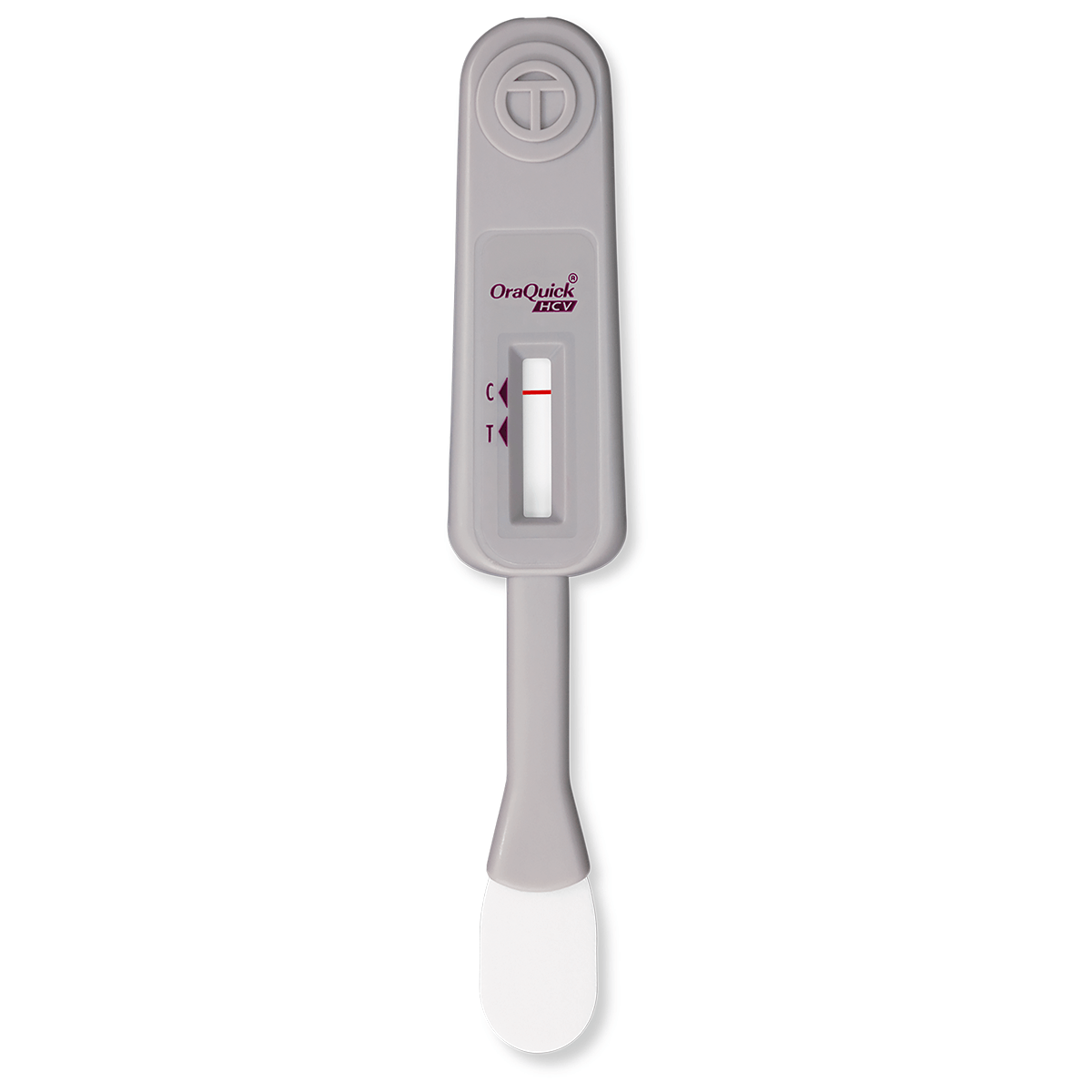
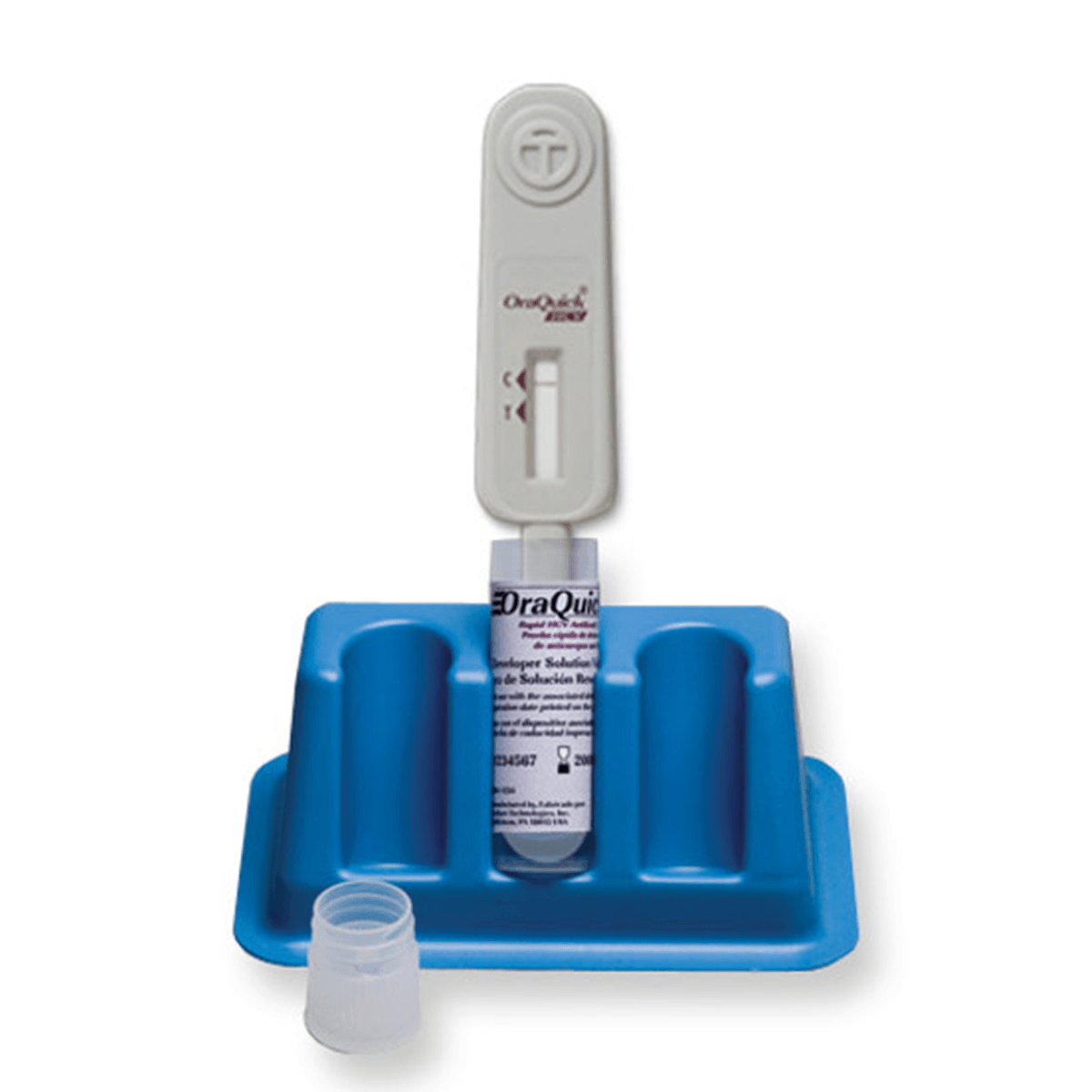
The OraQuick® HCV test is the first FDA approved test for detecting HCV antibodies in finger-stick and venipuncture whole blood. The simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes.
This is the initial laboratory test used for people with signs or symptoms, or who are at risk of having HCV infection.
The test result is used in combination with other clinical information and blood tests to aid in the diagnosis of individuals with signs or symptoms of hepatitis, or in individuals at risk for hepatitis C infection.
Product Specifications
| Tests/box | 25 |
| Detects | Multiple HCV genotypes |
| Sample type | Fingerstick and Venipuncture whole blood collection |
| Time to results | 20 minutes |
| Accuracy | Greater than 98% |
| Kit storage conditions | Room temperature (2°C to 30°C/36°F to 86°F) |
| Positive controls | Included |
| Negative controls | Included |
| CLIA complexity | Waived* |
| Certification | FDA |
Share